Chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction.[1] The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.[2]
Structure
Balancing chemical equationsEdit
Because no nuclear reactions take place in a chemical reaction, the chemical elements pass through the reaction unchanged. Thus, each side of the chemical equation must represent the same number of atoms of any particular element (or nuclide, if different isotopes are taken into account). The same holds for the total electric charge, as stated by the charge conservation law. An equation adhering to these requirements is said to be balanced.
A chemical equation is balanced by assigning suitable values to the stoichiometric coefficients. Simple equations can be balanced by inspection, that is, by trial and error. Another technique involves solving a system of linear equations.
Balanced equations are usually written with smallest natural-number coefficients. Yet sometimes it may be advantageous to accept a fractional coeficient, if it simplifies the other coefficients. The introductory example can thus be rewritten as
In some circumstances the fractional coefficients are even inevitable. For example, the reaction corresponding to the standard enthalpy of formation must be written such that one molecule of a single product is formed. This will often require that some reactant coefficients be fractional, as is the case with the formation of lithium fluoride:
Inspection methodEdit
The method of inspection can be outlined as setting the most complex substance's stoichiometric coefficient to 1 and assigning values to other coefficients step by step such that both sides of the equation end up with the same number of atoms for each element. If any fractional coefficients arise during this process, the presence of fractions may be eliminated (at any time) by multiplying all coefficients by their lowest common denominator.
- Example
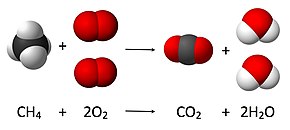
Balancing of the chemical equation for the complete combustion of methane
is achieved as follows:
- A coefficient of 1 is placed in front of the most complex formula (CH4):
- The left-hand side has 1 carbon atom, so 1 molecule of CO2 will balance it. The left-hand side also has 4 hydrogen atoms, which will be balanced by 2 molecules of H2O:
- Balancing the 4 oxygen atoms of the right-hand side by 2 molecules of O2 yields the equation
- The coefficients equal to 1 are omitted, as they do not need to be specified explicitly:
- It is wise to check that the final equation is balanced, i.e. that for each element there is the same number of atoms on the left- and right-hand side: 1 carbon, 4 hydrogen, and 4 oxygen.
System of linear equationsEdit
For each chemical element (or nuclide or unchanged moiety or charge) i, its conservation requirement can be expressed by the mathematical equation
where
- aij is the number of atoms of element i in a molecule of substance j (per formula in the chemical equation), and
- sj is the stoichiometric coefficient for the substance j.
This results in a homogeneous system of linear equations, which are readily solved using mathematical methods. Such system always has the all-zeros trivial solution, which we are not interested in, but if there are any additional solutions, there will be infinite number of them. Any non-trivial solution will balance the chemical equation. A "preferred" solution is one with whole-number, mostly positive[g] stoichiometric coefficients sj with greatest common divisor equal to one.
- Example
Let us assign variables to stoichiometric coefficients of the chemical equation from the previous section and write the corresponding linear equations:
All solutions to this system of linear equations are of the following form, where r is any real number:
The choice of r = 1 yields the preferred solution,
which corresponds to the balanced chemical equation:
Matrix methodEdit
The system of linear equations introduced in the previous section can also be written using an efficient matrix formalism. First, to unify the reactant and product stoichiometric coefficients sj, let us introduce the quantity
called stoichiometric number, which simplifies the linear equations to
where J is the total number of reactant and product substances (formulas) in the chemical equation.
Placement of the values aij at row i and column j of the composition matrix
and arrangement of the stoichiometric numbers into the stoichiometric vector
allows the system of equations to be expressed as a single matrix equation:
Like previously, any nonzero stoichiometric vector [νj], which solves the matrix equation, will balance the chemical equation.
The set of solutions [νj] to the matrix equation is called the kernel of the matrix [aij]. To have a kernel with nonzero vectors, the columns of the composition matrix must not be linearly independent and its kernel will have a positive dimension JN. The problem of balancing a chemical equation then becomes the problem of determining the JN-dimensional kernel of the composition matrix. It is important to note that only for JN = 1 will there be a unique preferred solution to the balancing problem. For JN > 1 there will be an infinite number of preferred solutions with JN of them linearly independent. If JN = 0, there will be only the unusable trivial solution, the zero vector.
Techniques have been developed[6][7] to quickly calculate a set of JN independent solutions to the balancing problem, which are superior to the inspection and algebraic method in that they are determinative and yield all solutions to the balancing problem.
- Example
Let us take the same chemical equation again and write the corresponding matrix equation:
Its solutions are of the following form, where r is any real number:
The choice of r = 1 and a sign-flip of the first two rows yields the preferred solution to the balancing problem:
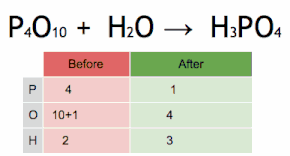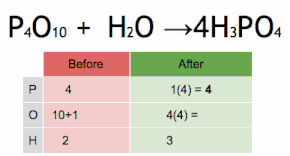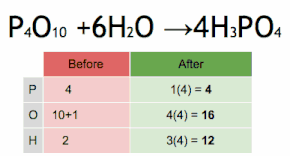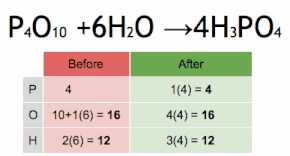


Comments
Post a Comment